MRI Safety Week (commencing 23 July 2018) promotes excellence in MRI safety. Sadly, this week marks the 17th anniversary of the tragic 2001 MRI accident that resulted in the preventable death of Michael Colombini, age 6, who died when a portable steel oxygen cylinder was brought into the MRI room during his MR scan.
To promote best practice and excellence in MRI Safety week, a series of short advice sheets will be published by the BIR which have been put together by the BIR Magnetic Resonance Special Interest Group with support and advice from the IPEM MR Special Interest Group.
Overview of MR Safety Week 2018: Assessing MR Conditions for Implanted Devices.
When considering a request for an MRI scan for a patient with an implanted device there is a need to determine whether the scan can safely proceed with the device present. Ideally, the manufacturer should provide a label for the device. If it is labelled MR Conditional, then the manufacturer should provide a further set of specified conditions for the device.
MR Safety Labelling
Ideally, an implant will be either:
- MR Safe
- MR Conditional with the conditions clearly stated by the manufacturer and able to be met in the scanner.
- MR Unsafe
In the first two cases a scan may proceed without further investigation.
Sometimes the situation is not as straightforward. For example, it may be the case that:
- You cannot meet the conditions specified by the manufacturer of a MR Conditional device.
- The device manufacturer labels the device as MR Unsafe, but you wish to consider a scan.
- The device manufacturer doesn’t provide clear or perhaps any MR safety advice.
- You cannot get information about the device manufacturer.
There is always a degree of risk associated with any clinical procedure. There may be situations where the clinical need for the MRI scan outweighs the risks associated with not having reassurance from the device manufacturer that it is safe to scan a patient with the implanted device. It may still be possible to safely proceed with a scan, but further investigation is required.
BIR Advice Sheets below
During this week a series of short advice sheets will be released that will briefly describe commonly encountered MR Conditions, why they are important and where to find some further information. On the final day, the situation described above where further investigation is required will be explored.
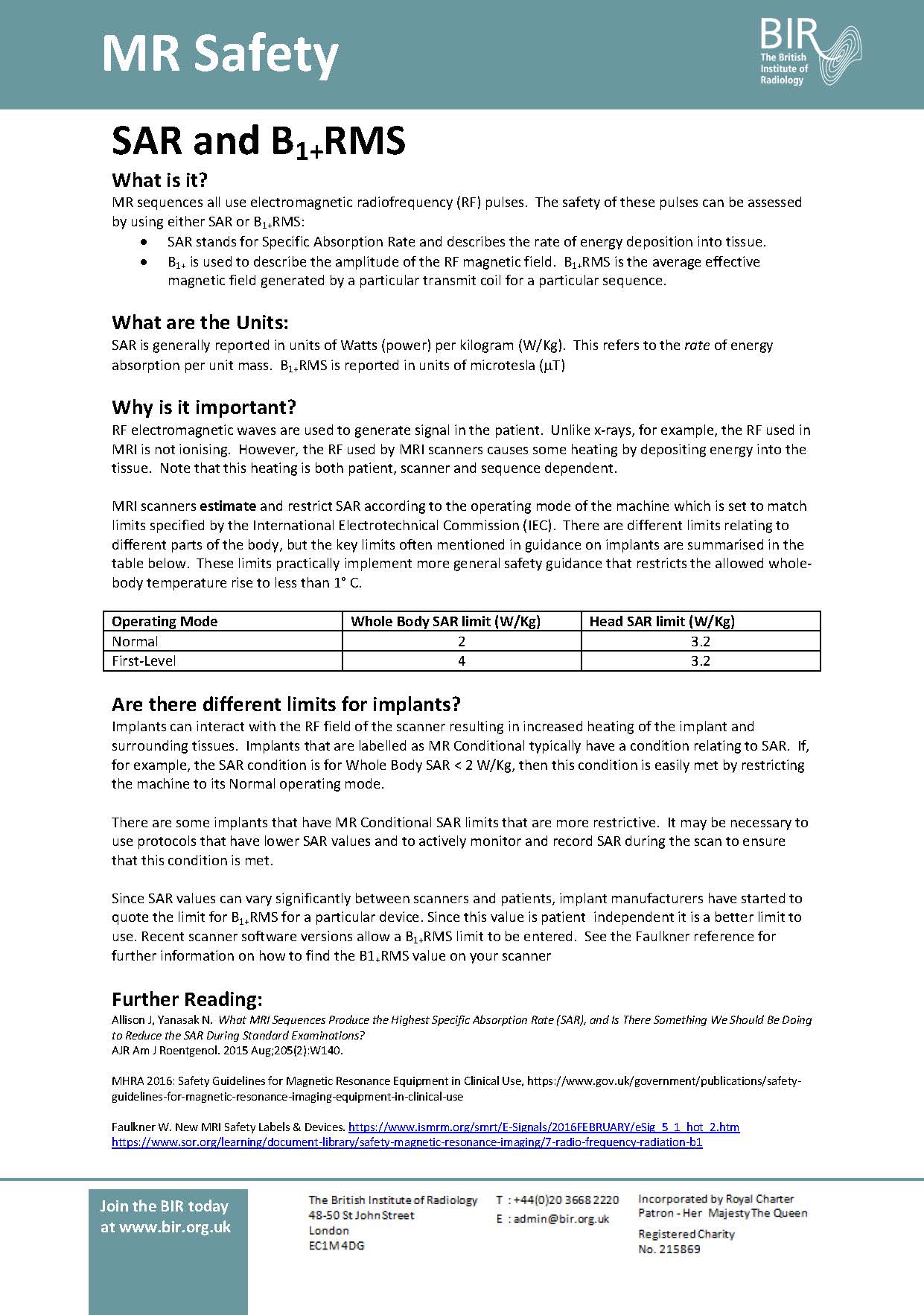

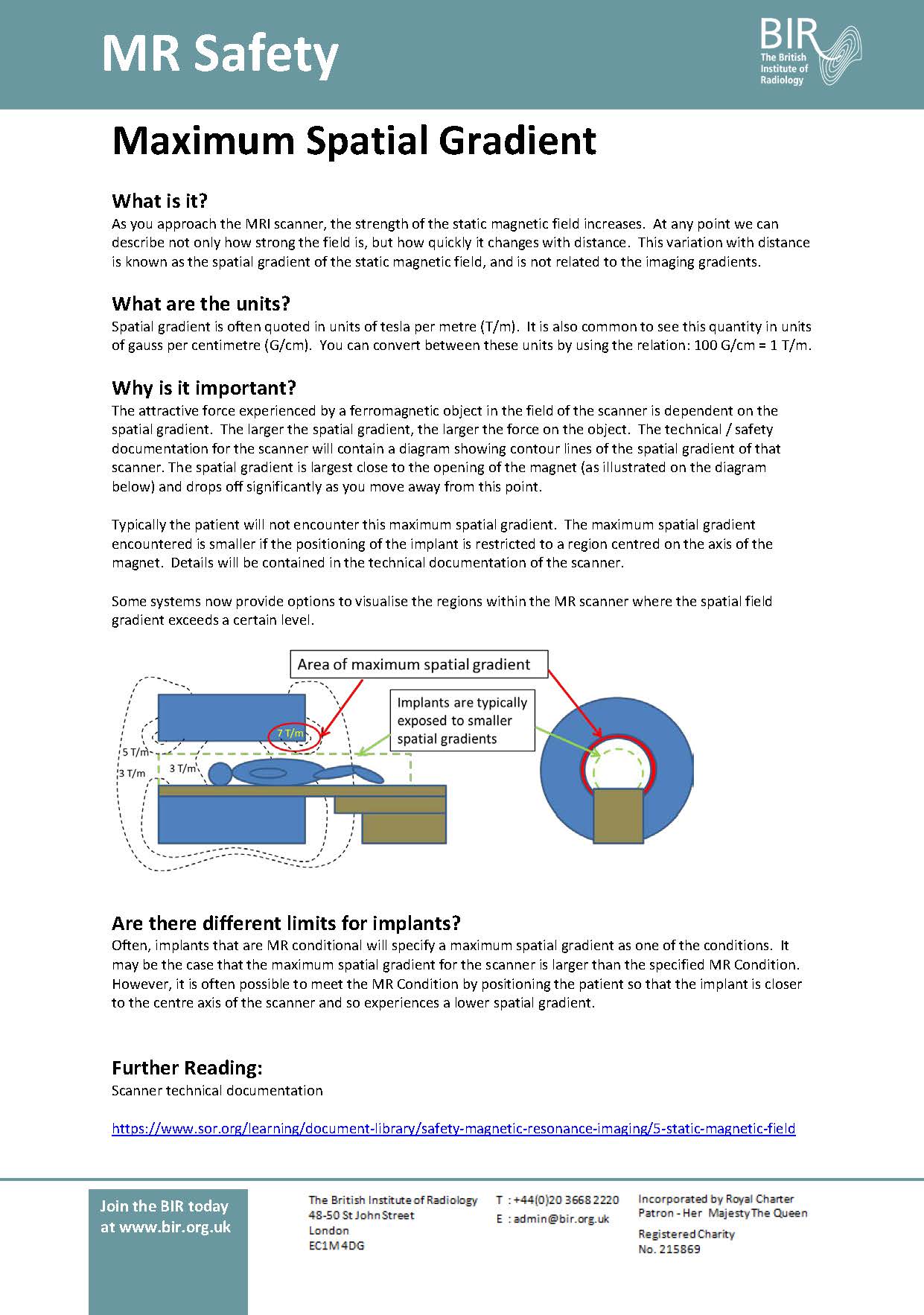
Day 1: SAR and B+RMS Day 2: Static Magnetic Field Day 3: Spatial Gradient


Day 4: Magnetic Field Gradients Day 5: Scanning without manufacturer's approval of MRI Safety
Image courtesy of InHealth
Find out more about the BIR MR Special Interest Group here
Register to find out more about the MR SIG here